
ACoMedIn
FZ: 16KN053228
Corrosion-free marking of medical instruments
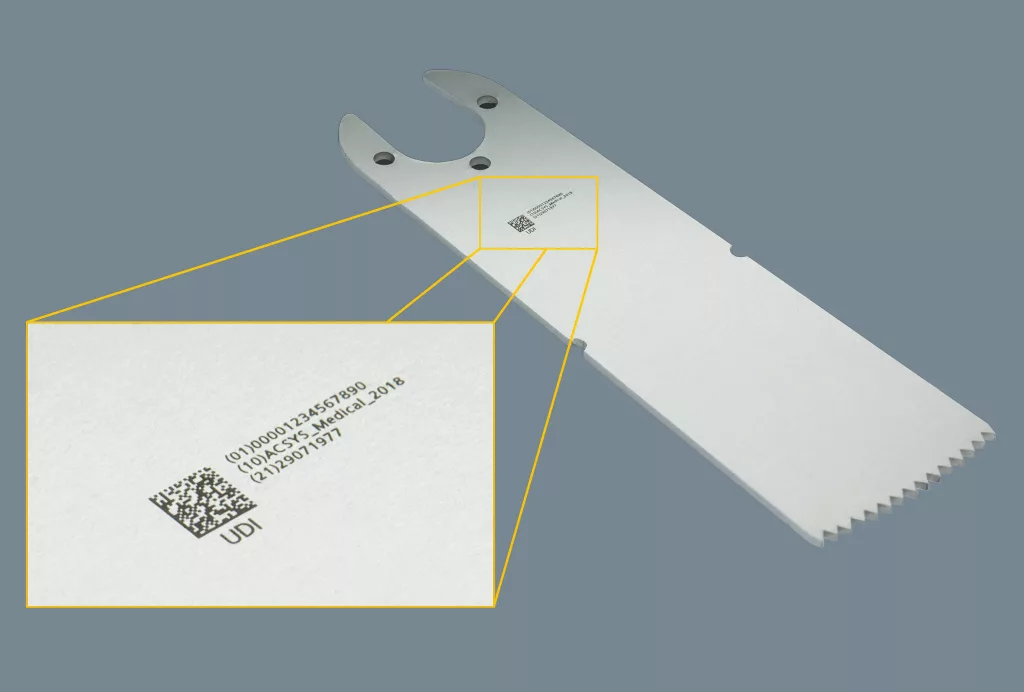
From 2020, all medical instruments must be labeled in accordance with the FDA’s UDI guidelines and the future guidelines of the European Union’s new Medical Device Regulation in order to enable the classification and traceability of medical instruments. Conventional laser marking damages the chromium oxide layer that protects against corrosion and therefore requires subsequent passivation. In addition, material abrasion or the influence of heat can cause cracks in which germs can colonize and resist cleaning.
ACSYS aims to develop suitable laser marking processes that guarantee corrosion resistance over the service life of the medical instrument, even when cleaning in highly alkaline media.







Your ACSYS laser expert
Do you have any questions?
I am happy to help.
Ardalan Masoumi
Business Development Manager
Telephone: +49 7154 80875 531
E-Mail: a.masoumi@acsys.de
